MGH Biostatistics leads and enables research in the design and conduct of clinical trials, observational data methods, and methods for precision medicine applications. Collectively, the MGH Biostatistics faculty members have expertise in methods for clinical trials and observational studies, clustered and repeated measures data, missing data, time-to-event outcomes, causal inference, Bayesian analysis, machine learning, genetic epidemiology, -omics applications, implementation science, and precision medicine.
In 2021, MGH Biostatistics faculty contributed to 297 published manuscripts and collaborated on 186 research projects across dozens of Divisions and Departments. The examples below highlight some of our major collaborative research efforts.
Select Collaborations
MGH Biostatistics partners with the Sean M. Healey & AMG Center for ALS at Mass General and Tackle ALS to lead the first platform trial initiative for amyotrophic lateral sclerosis (ALS, Lou Gehrig’s disease). ALS is a neurological disease with limited treatment options.
A “platform trial” is a clinical trial in which multiple treatments are evaluated simultaneously using specialized statistical tools. New drug regimens can be added as they become available, decreasing or eliminating the lengthy start-up and execution times of traditional trial models. The platform model, already proven successful in the cancer field, will greatly accelerate therapy development of effective and breakthrough treatments for people with ALS by allowing investigators to test more drugs, increase patient access to trials and reduce the cost by quickly and efficiently evaluating the effectiveness of multiple therapies. The focus is on the disease, rather than any individual experimental agent, and the platform remains open long-term until successful cures are found.
MGH biostatisticians play prominent roles in a wide range of clinical and translational research at the MGH Cancer Center (MGHCC) and through the Biostatistics Core of the Dana-Farber/Harvard Cancer Center (DF/HCC).
- We have been instrumental in developing and reporting clinical trials for adult and pediatric cancers, ranging from early phase to randomized studies in local and multi-center protocols. Among the contributions to advances in novel and innovative therapies, we have maintained notably long-term collaborations in radiation oncology, especially the Proton Research Program.
- In addition to the therapeutic studies, we are involved in clinical trials initiated by the Cancer Outcomes Research & Education Program (CORE), as emphasis increasingly shifts to the quality of cancer care and the relevance of patient-oriented outcome. We also conduct biostatistics clinics as part of the Annual Workshop on Methods in Supportive Oncology Research hosted by the CORE leadership for junior investigators from around the world.
- Our contributions to translational research involve the design and analysis of preclinical models as well as human studies, including discovery and validation of prognostic and predictive biomarkers. In particular, MGH biostatisticians play key roles in the DF/HCC SPOREs for Glioma, ovarian, and gastrointestinal cancers.
- MGH Biostatistics has a robust research program in early detection of cancer, originally focused on ovarian cancer and recently expanded to include renal cell carcinoma (kidney), hepatocellular carcinoma (liver), lung cancer, and prostate cancer. NCI-sponsored research includes biomarker discovery and validation, algorithm development, and clinical diagnostic and screening studies for early detection of ovarian cancer in the Early Detection Research Network and lung cancer screening through the Liquid Biopsy Consortium. Additionally, we collaborate with the Center for Innovation in Early Cancer Detection at MGH.


 Researchers from Mass General Brigham have been selected by the National Institutes of Health (NIH) to serve as the Data Resource Core for RECOVER, an important research initiative to help the country rapidly improve understanding of recovery after COVID-19 infection and to prevent and treat the long-term complications, collectively referred to as Post-Acute Sequelae of SARS-CoV-2 infection (PASC). The magnitude of the public health impact of these sequelae is currently unknown but potentially large given the numbers of individuals across the age spectrum who have been and will be infected with SARS‐CoV‐2. It is a public health priority that we better understand and develop strategies that enable rapid innovation, evolution, and adaptation in the prevention and treatment of PASC.
Researchers from Mass General Brigham have been selected by the National Institutes of Health (NIH) to serve as the Data Resource Core for RECOVER, an important research initiative to help the country rapidly improve understanding of recovery after COVID-19 infection and to prevent and treat the long-term complications, collectively referred to as Post-Acute Sequelae of SARS-CoV-2 infection (PASC). The magnitude of the public health impact of these sequelae is currently unknown but potentially large given the numbers of individuals across the age spectrum who have been and will be infected with SARS‐CoV‐2. It is a public health priority that we better understand and develop strategies that enable rapid innovation, evolution, and adaptation in the prevention and treatment of PASC.
As the Data Resource Core for RECOVER, a four-year, multimillion dollar project, MGH Biostatistics and MGB will provide expertise on study design and facilitate the collection and analysis of complex data across multiple cohort studies. The team is led by Andrea Foulkes, ScD, Chief of MGH Biostatistics, Elizabeth Karlson, MD, MS Director of Rheumatic Disease Epidemiology at Brigham and Women’s Hospital, and Shawn Murphy, MD, PhD, Chief Research Information Officer at Mass General Brigham, and includes complementary teams from Harvard Medical School and Harvard T.H. Chan School of Public Health.

The study assesses the positive predictive value of an irregular heart rhythm detection during the ECG patch monitor period and also examines the validity of irregular pulse tachograms during subsequent heart rhythm detections, self-reported AF diagnoses and treatments, and relations between irregular heart rhythm detections and AF episode duration. The Fitbit Heart Study results will provide critical insights into the use of consumer wearable technology for AF detection and for characterizing the nature of AF episodes detected using consumer-based PPG technology. Advancing research on innovative and accessible technology, like wearable Fitbit devices, will lead to more tools that help improve health outcomes and reduce the impact of AF on a large scale.
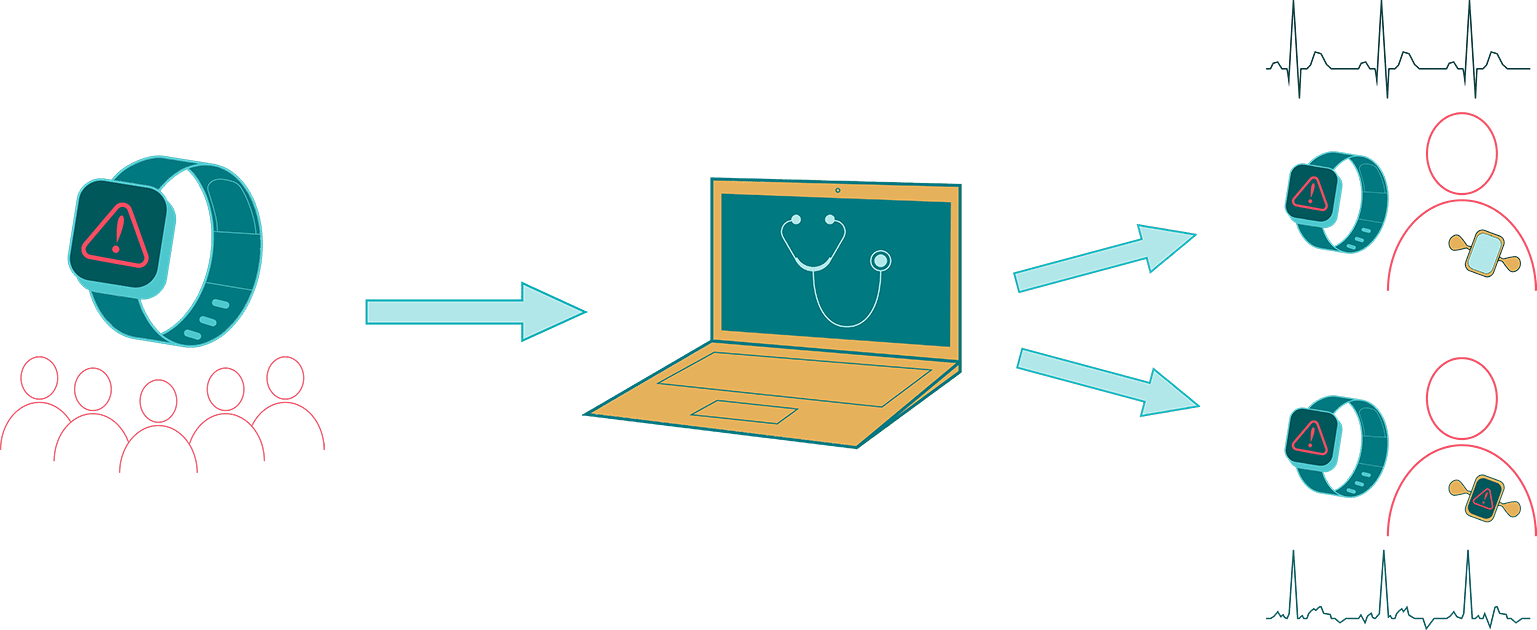

In 2020, the PETAL Network redirected some of its effort to COVID-19 and completed a randomized controlled clinical trial of hydroxychloroquine in hospitalized patients with COVID-19. It also launched multicenter observational retrospective and prospective studies designed to collect comprehensive data on hospitalized patients with COVID-19, including imaging, biospecimens, and long-term outcomes. In addition, the PETAL Network is a collaborator in both the ACTIV-3 and ACTIV-4 platform trials.
Featured Publication